In June 2017, HIQA commenced work on a health technology assessment (HTA) in relation to proposed changes to the national human papillomavirus (HPV) immunisation programme. Following a formal request from the Department of Health, HIQA agreed to undertake the HTA. This HTA aims to establish the clinical and cost-effectiveness of extending the current immunisation programme, which offers HPV vaccination to all girls in their first year of secondary school (12 to 13 year olds), to a programme that also offers the vaccination to boys.
HTA of extending the HPV vaccination to boys
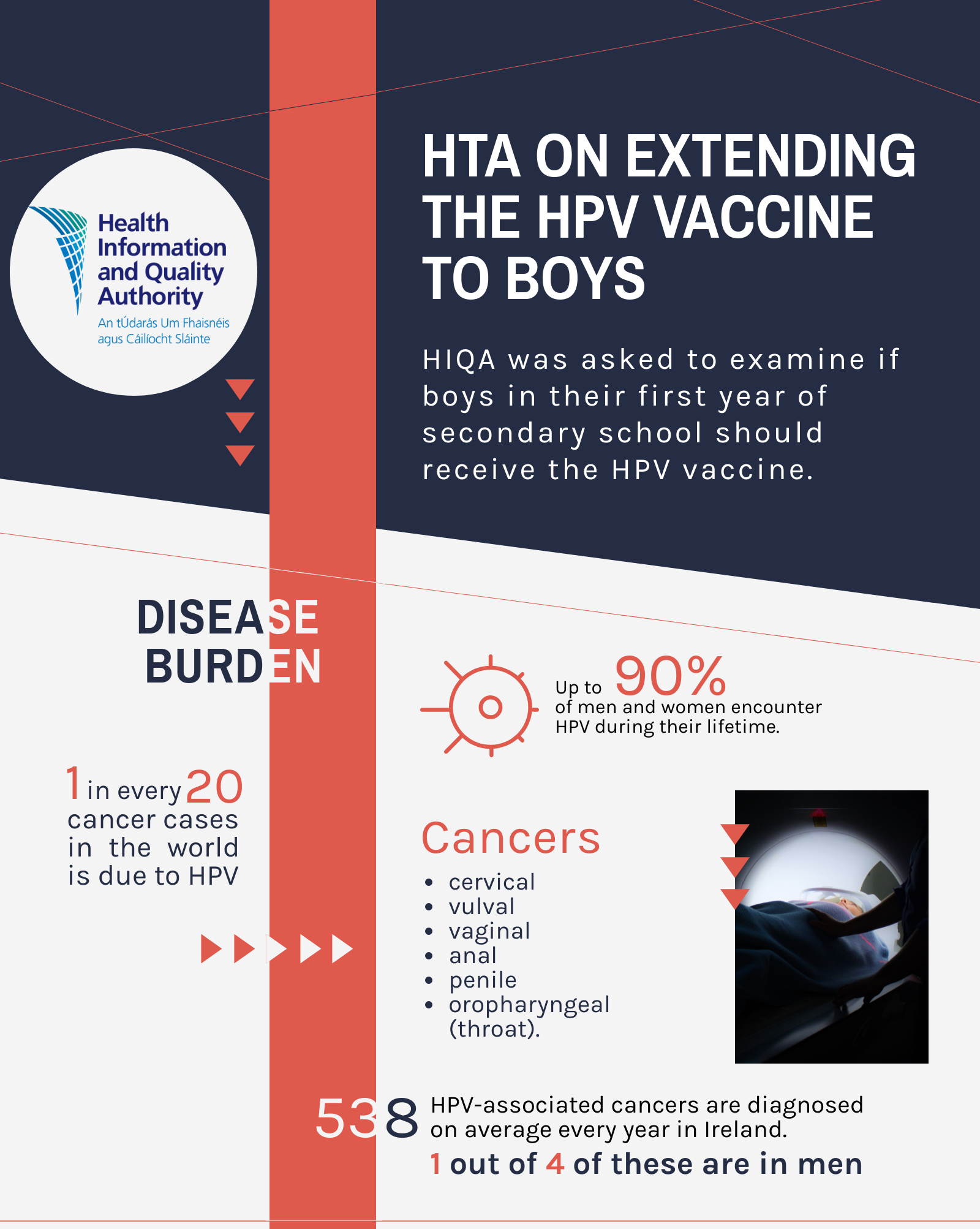
Human papillomavirus (HPV) is the most common viral infection of the reproductive tract and is the cause of a range of conditions in both males and females, including a range of cancerous and precancerous lesions and anogenital warts. Although the majority of HPV infections do not cause symptoms and resolve spontaneously, persistent infection with HPV may result in disease. The burden of HPV-related disease is substantial; HPV is responsible for approximately 1 in every 20 cases of cancer across the world.
Cervical cancer is the most common cancer caused by HPV. The virus is also linked to cancers of the vulva, vagina, anus, penis and an area at the back of the throat known as the ‘oropharynx’. HPV also causes warts in the anus and genital areas of both men and women. Every year, 538 cancers associated with HPV are diagnosed in Ireland. There are two ways to help prevent cancer associated with HPV infection: primary prevention through vaccination and in the case of cervical cancer, secondary prevention through screening. Three vaccines are available in Ireland that offer protection against HPV infection: the 2-valent vaccine that protects against two HPV types, the 4-valent vaccine that protects against four HPV types and 9-valent vaccine that protects against nine HPV types.
This research was carried out in accordance with HIQA’s guidelines for the conduct of HTAs. In summary, the following took place:
- The Terms of Reference of the HTA were agreed between HIQA and the Department of Health.
- An Expert Advisory Group was convened, with representation from health policy decision-makers, clinicians, professional bodies, the national parents’ council post primary, and experts in the fields of vaccinology, health services research, ethics and economic evaluation. An Evaluation Team was appointed comprising HIQA staff.
- The epidemiology of HPV infection and the burden of HPV-related disease in Ireland were assessed.
- A systematic review and meta-analysis of randomised controlled trials (RCTs) was carried out to summarise the available evidence on the efficacy of the 4- and 9-valent HPV vaccines.
- A systematic review of time-trend observational studies was updated to summarise the population-level effect of HPV immunisation programmes on HPV-related disease.
- A systematic review of systematic reviews was undertaken to assess the safety of the HPV vaccine, as well as retrieving Irish safety data and reviewing other key narrative reviews and independent expert analyses. A systematic review was undertaken to summarise the available cost-effectiveness evidence of gender-neutral HPV vaccination.
- An economic model was adapted to the Irish setting to estimate the cost-effectiveness of different HPV vaccination strategies for the target population (12 and 13 year old boys and girls).
- A budget impact analysis reporting the incremental costs associated with the proposed changes to the HPV immunisation schedule over a one and five-year time horizon was performed from the perspective of the public health and social care system.
- An analysis of the organisational, social and ethical implications was undertaken with a view to identifying broader considerations that may influence decision-making.
- The complete draft report was reviewed by the Expert Advisory Group, before being made available for public consultation, to give interested parties an opportunity to comment on the draft before it was finalised.
- A final draft of the report, including a report on the results of the public consultation, was submitted to the Board of HIQA for approval.
- Following its approval, the completed assessment was submitted to the National Immunisation Advisory Committee (NIAC), the National Immunisation Office (NIO), the Department of Health and the Minister for Health as advice, and published on the HIQA website.
- The burden of HPV-related disease is substantial in Ireland, with an average of 538 HPV-associated cancers diagnosed per year in men and women.
- A systematic review of efficacy demonstrated that HPV vaccines are highly efficacious in preventing HPV infection and its sequelae in adults. Evidence of efficacy in pre-adolescents was confirmed through immunobridging studies, whereby younger populations demonstrated a superior immune response to adults and males demonstrate a superior immune response to females.
- The high efficacy observed in clinical trials is supported by observational studies, whereby the introduction of HPV immunisation programmes has led to significant reductions in HPV-related disease on a population level.
- A large volume of evidence demonstrates the overall safety of HPV vaccines. An overview of reviews, encompassing data from over 70,000 trial participants and over 20 million individuals in observational studies, did not identify an increased rate of serious adverse events in recipients of HPV vaccines compared with placebo.
- A change to the HPV immunisation programme should include adoption of the 9-valent vaccine. A gender-neutral 9-valent programme was estimated to be more effective and more costly than the girls-only 9-valent alternative. In light of the conservative assumptions regarding price, uptake rate and exclusion of protection versus oropharyngeal cancer in the base case as well as the proposed decrease in the discount rate from 5% to 4%, it is likely that gender neutral 9-valent vaccination would be considered cost-effective at €45,000 per quality-adjusted life year (QALY).
- HPV vaccination of boys provides direct protection against HPV-related disease to boys. It also provides indirect herd protection to girls who have not been vaccinated. Other important factors to consider include the additional protection provided by a gender neutral programme to vulnerable groups (for example, men who have sex with men) and the potential to improve the resilience of the immunisation programme to fluctuations in vaccine uptake and to the movement of individuals into and out of the country.
