The health technology assessment (HTA) was requested by the HSE’s National Clinical Programme for Stroke to evaluate the use of endovascular therapy using mechanical thrombectomy devices for acute ischaemic stroke. The assessment was carried out by an Evaluation Team from the HTA Directorate in HIQA. A multidisciplinary Expert Advisory Group was established to provide advice on the assessment.
HTA of Mechanical Thrombectomy for Stroke
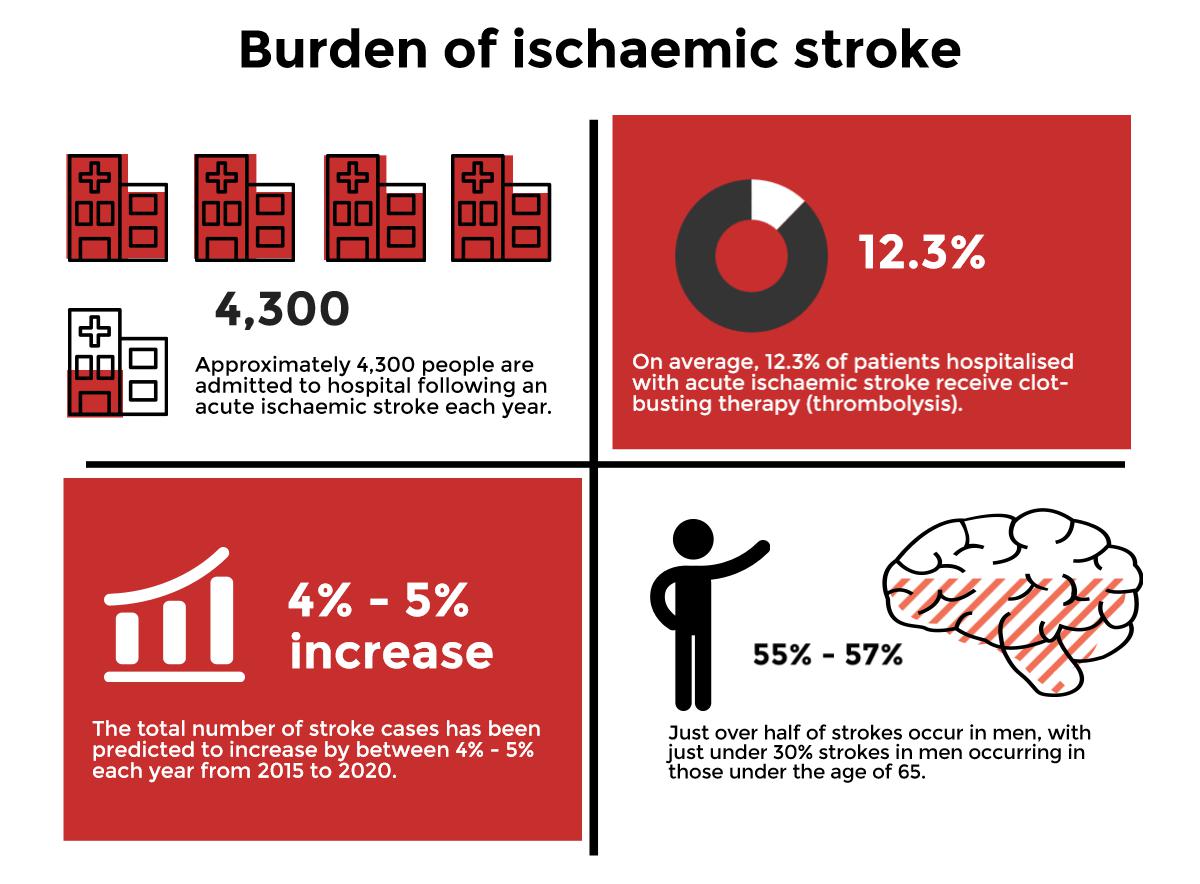
To inform decisions around a national emergency endovascular service using mechanical thrombectomy devices for the management of acute ischaemic stroke.
The Health Information and Quality Authority (HIQA) received a request from the Health Service Executive (HSE)’s National Clinical Programme for Stroke to examine the clinical effectiveness, cost-effectiveness, budget impact and the organisational and ethical implications of mechanical thrombectomy for the management of acute ischaemic stroke
This health technology assessment (HTA) was initially undertaken as a pilot rapid assessment through HIQA’s work with the European Network for Health Technology Assessment (EUnetHTA). Co-authored by HTA colleagues from Germany, the rapid assessment included a systematic review of the efficacy and safety of mechanical thrombectomy for selected patients. The EUnetHTA report, published in December 2015, concluded that endovascular therapy using mechanical thrombectomy is a safe and effective adjunct to standard medical care for selected patients. In accordance with the terms of reference, the EUnetHTA report was subsequently updated and adapted to include data on the burden of stroke in Ireland, a systematic review of the cost-effectiveness literature, an economic model to estimate the cost-effectiveness and budget impact, as well as a review of the organisational and ethical implications in the context of the Irish healthcare system.
Use of a systematic review approach allows us to identify, appraise and synthesise the best available evidence and is done in line with international best practice. An Expert Advisory Group (EAG) was convened to inform and guide the process, and provide expert advice, information and access to data, where appropriate. The EAG comprised representation from relevant stakeholders including the HSE’s National Clinical Programme for Stroke, clinicians with specialist expertise, patient representation, representation from the National Ambulance Service and international HTA experts.